By Doris Obinna
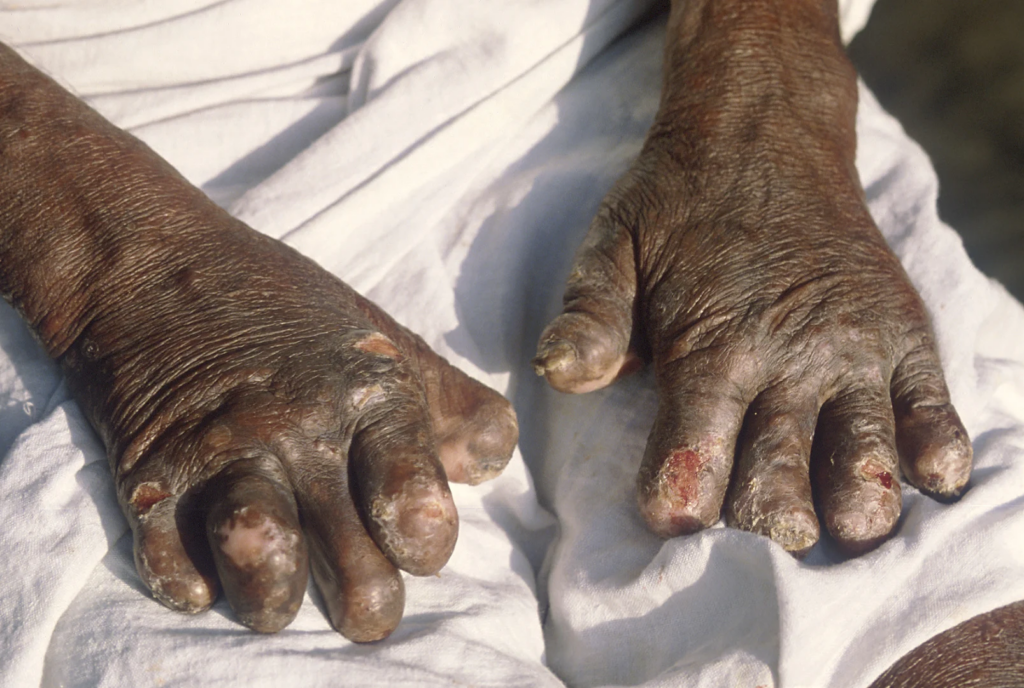
The National Agency for Food and Drug Administration and Control (NAFDAC) has responded to a recent British Broadcasting Corporation (BBC) report that suggested the agency was responsible for delaying the importation of vital leprosy drugs into Nigeria.
The BBC report, titled “Vital leprosy drugs due in Nigeria after year delay,” alleged that the World Health Organization (WHO) had urged NAFDAC to lift its testing policy to allow the importation of anti-leprosy medicines needed by patients. However, NAFDAC has refuted these claims, stating that the delay was due to the failure of the manufacturer to obtain the necessary quality assurance certification from Indian regulatory authorities.
In a press statement signed by the Director General, Prof. Mojisola Adeyeye, NAFDAC emphasised its commitment to ensuring the safety, efficacy, and quality of medicines imported into Nigeria. The agency explained that its Clean Report of Inspection and Analysis (CRIA) Scheme, in place since 2002 and strengthened in 2020, requires medicines from high-risk countries like India and China to undergo rigorous testing before they are allowed into Nigeria.
“One key requirement of this process is the submission of a Certificate of Pharmaceutical Products (CoPP), an international quality assurance document endorsed by the WHO.”
NAFDAC stated that the manufacturer of the leprosy drug Rifampicin failed to provide the necessary CoPP certification from Indian regulatory authorities, prompting the WHO to request a waiver of the requirement.
In response, NAFDAC sought laboratory test results from one of its approved CRIA laboratories in India to verify the quality of the shipment. After confirming that the drug met safety and efficacy standards, NAFDAC approved its release for export to Nigeria.
The agency reiterated its commitment to protecting public health and assured Nigerians that it would continue working with local pharmaceutical manufacturers to reduce dependence on imported medicines.
“NAFDAC will continue to ensure that only quality, safe, and efficacious medicines are available for distribution, sale, and use within Nigeria,” stated Adeyeye.
The statement further reads: “NAFDAC has long enforced stringent regulations on drug imports to prevent the circulation of substandard or counterfeit medicines. The CRIA scheme ensures that pharmaceutical products from high-risk countries meet international Good Manufacturing Practices (GMP) before they reach Nigerian markets.
“With the increasing push for local drug production, NAFDAC is collaborating with domestic pharmaceutical firms to strengthen Nigeria’s pharmaceutical industry and reduce reliance on foreign imports.”
While concerns had been raised over delays in drug imports, NAFDAC insists that its policies are designed to protect the public from potentially harmful or ineffective medications.